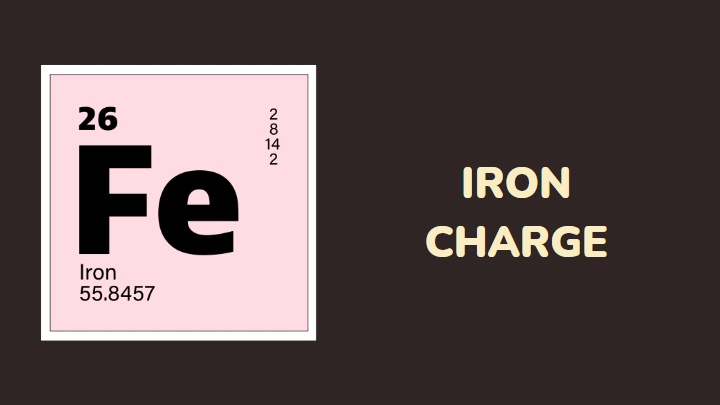
A large majority of transition metals have a high tendency of exhibiting multiple charges. Iron, one of the first transition metals on the periodic table, is the focus of this article, and I will be discussing its charge.
Iron can exist as a +2, +3, or +6 ion. The +2 and +3 charges are the most commonly seen in complexes. The charge an element exhibits depend on how many electrons it gained or lost to form a compound with other elements.
Let’s look at why iron can exhibit multiple charges.
Properties of iron
- Iron is a transition metal in group 8 with an atomic number of 26 and mass number of 55.8
- It is a lustrous metal with a grayish tinge
- Iron has an electronic configuration of [Ar]3d64s2
- Iron is the second most abundant metal on the earth’s crust. It takes up about 5% of the Earth’s surface
- At the same time, iron makes up 35% of the Earth’s core
- The melting point and boiling point of iron are 2,800°F (1,538 °C) and 5,432°F (3,000 °C) respectively
- Iron exists in its free form and as an alloy with metals such as nickel. Other forms in which iron exists are hematite or ferric oxide (Fe2O3) and magnetite or triiron tetroxide (Fe3O4)
What is the charge of iron?
Iron exhibits multiple charges. +2 and+3 are the most common charges. These multiple oxidation states are commonly seen in the two iron oxides, FeO and Fe2O3.
Iron (II) oxide, FeO turns green when it dissolves in water. Iron (III) oxide, Fe2O3, turns reddish-brown when it dissolves in water.
What is the charge of iron in potassium ferrate?
The charge of iron in potassium ferrate, K2FeO4 is +6.
The charge of potassium is +1 and the charge of oxygen is -2.
2(1) + Fe + 4(-2) = 0
2 + Fe – 8 = 0
Fe – 6 = 0
Fe = +6
Why does iron have a +3 charge?
When iron loses three electrons, it gains a +3 charge.
FAQs
What is the charge of Fe in FeSO4?
The charge of Fe in FeSO4 is +2. The respective oxidation states of S and O are +6 and -2. The overall charge of the compound is 0.
Fe + 6 + 4(-2) =0
Fe + 6 – 8 = 0
0 = Fe – 2
Fe = +2
What is the charge of Fe in Fe(CN)6?
The charge of Fe in Fe(CN)6 is +6. The charge of CN is -6. The overall charge of the compound is 0.
Fe + 6(-1) = 0
0 = Fe – 6
Fe = +6
What is the charge of Fe in [Fe(CN)6]4-?
The charge of Fe in [Fe(CN)6]4- is +2.
In a mathematical expression, substitute -1 as the oxidation number for CN.
Fe + 6(-1) = -4
Fe – 6 = -4
-4 + 6 = Fe
Fe = +2
Conclusion
Depending on the type of compound iron forms, you will most likely find it with a +2 or +3 charge. It forms amphoteric oxides and can exhibit multiple oxidation states such as -4, -2, -1, 0, +1, +2, +3, +4, +5, +6, and+7.
You can also learn about the charge of cesium.
Thanks for reading.