One definition of matter that cuts across all is “it is a substance that occupies space, has mass, and can remain unchanged or unmoved if left alone”. Moreover, all forms of matter possess physical and chemical properties that separate one from the other.
Physical and chemical properties have significant differences which is what this article looks into. To easily understand this differentiation, physical properties do not affect the chemical identity of the material, but chemical properties affect the chemical identity of the material.
What is matter?
Matter makes up every observable material in the universe. It has mass, occupies space, and is naturally in a state of inertia.
The definitions of matter seem to imply that anything can count as matter. This, however, is not true. Photons, heat, and microwaves are not examples of matter because they do not have mass and cannot occupy space.
Matter comprises atoms which are a combination of protons, electrons, and neutrons. Furthermore, atoms are building blocks of molecules and elements.
Atoms and molecules of two or more elements come together to form a compound that may or may not look anything like its constituent atoms or molecules.
What are the physical properties of matter?
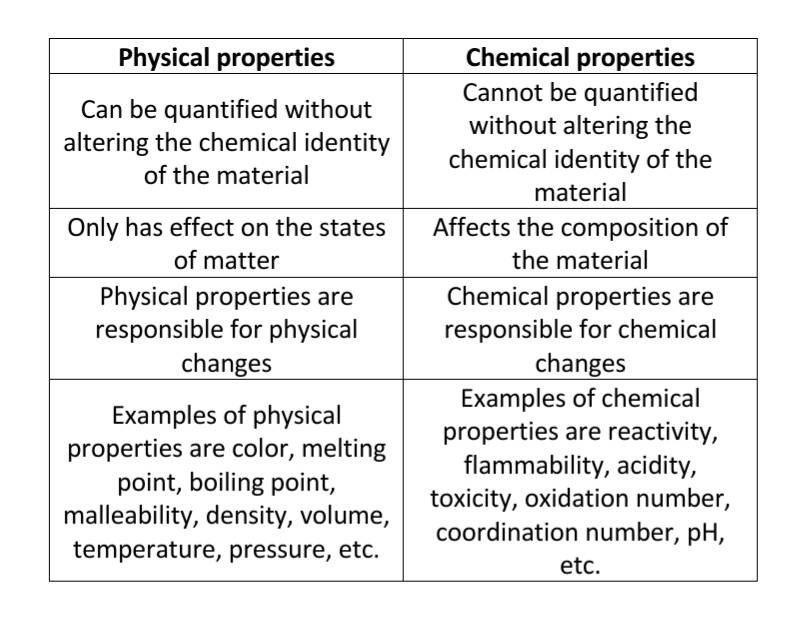
Physical properties are properties of matter that can be quantified without altering the chemical composition of the material.
These properties describe the overall physical appearance of the material. Physical properties are either intensive or extensive. Intensive physical properties do not factor the amount of the material while extensive physical properties factor the amount of the material.
Furthermore, intensive properties do not change when the quantity of the material changes. Some of these properties are color, density, melting point, and boiling point.
Conversely, extensive properties change with the quantity of the material. Examples are mass, volume, width, and length.
In addition, physical properties influence physical change (a change that alters only physical appearance) such as the states of matter.
What are the chemical properties of matter?
Chemical properties of matter are those characteristics that can be quantified only after altering the chemical composition of the material. It also refers to the tendency of that material to undergo chemical changes.
Chemical properties are quantifiable when the material undergoes chemical reactions. For example, one of the chemical properties of iron is seen when it reacts with oxygen and begins to rust, the formation of iron oxide.
Also, the chemical properties of different materials show through their reaction with acids, bases, water, flame, heat, and a catalyst.
Examples of chemical properties are reactivity, flammability, electronegativity, oxidation state, acidity, and enthalpy of combustion.
Tabular differences between chemical and physical properties
What are the factors that influence the physical properties of matter?
Temperature and pressure are two factors that affect the physical properties of a material. When the temperature or pressure changes, it alters the state of matter of that material.
What are the factors that influence the chemical properties of matter?
The chemical properties of matter can change when the melting point, boiling point, solubility, and number of electrons in the atom change.
FAQs
What is the fourth state of matter?
The fourth state of matter is plasma, ionized gas. Plasma is made of equal amounts of positively and negatively charged particles. It is the most abundant state of matter in the universe. It is what makes up the stars and sun.
What are the physical reactions that matter undergoes?
Most physical reactions of matter are reversible as they do not affect the composition of the material. These reactions include vaporization, freezing, condensation, dissolving, burning, chopping wood, melting ice, crushing a can, and mixing sand and water.
What are the chemical reactions that matter undergoes?
The chemical reactions matter changes its state, color, smell, and appearance. Some of these reactions include bond formation, bond dissolution, sublimation, evaporation, burning, combustion, electroplating, and precipitation.
Conclusion
Matter has both physical and chemical properties. The major difference between these properties is how one influences the chemical composition of the material.
You can remember it this way: chemical properties have a direct relationship with the chemical bonds within the material. On the other hand, physical properties are merely superficial.
Thanks for reading.
Check Gezro for more informational chemistry articles like this.