SO2, a compound composed of sulfur and oxygen atoms, has a strong presence in industrial processes, volcanic eruptions, and atmospheric emissions.
Understanding the polarity of SO2 is paramount for deciphering its behavior in diverse environments and its profound impact on human health and the environment.
In this article, you will discover the factors that shape the polarity of SO2, how it influences its physical and chemical properties as well as its implications in atmospheric chemistry, pollution control, and industrial applications.
What is SO2?
SO2, known as sulfur dioxide, is a chemical compound composed of sulfur and oxygen atoms.
It is a colorless gas with a sharp, pungent smell, often described as resembling the smell of burnt matches.
SO2 is produced primarily by the burning of sulfur-containing fuels like coal and oil, as well as during volcanic eruptions and certain industrial processes.
It plays a significant role in various environmental processes. In addition to its natural sources, SO2 is also generated by human activities such as industrial production and transportation.
Is SO2 polar or non-polar?
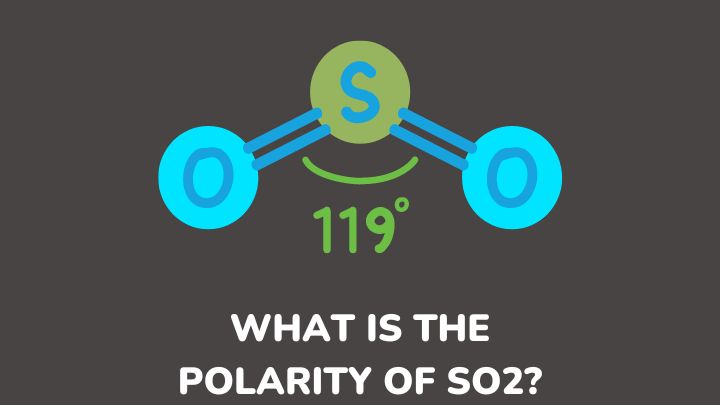
SO2 is a polar molecule with a bent structure. Many factors explain the polarity of SO2. These factors are discussed below.
Understanding the polarity of SO2
1. Molecular structure of SO2
In the Lewis structure of SO2, sulfur is bonded to two oxygen atoms by double bonds, and it has one lone pair of electrons. This arrangement gives us insight into how the atoms are connected within the molecule.
Next, the molecular geometry of SO2 is important. It has a bent shape due to the lone pairs of electrons on the sulfur atom. These lone pairs repel the bonded electron pairs, causing the molecule to bend.
Furthermore, the concept of resonance hybridization helps us understand the electronic structure of SO2.
It suggests that the actual structure of the molecule is a blend of different resonance structures, where electrons are delocalized between the sulfur and oxygen atoms.
2. Electronegativity difference
Electronegativity measures how strongly atoms attract electrons. In the case of SO2, sulfur (S) and oxygen (O) have different electronegativities.
Oxygen is more electronegative than sulfur, with an electronegativity value of approximately 3.44 for oxygen and 2.58 for sulfur, based on the Pauling scale. This means oxygen attracts electrons more strongly.
This difference in electronegativity between sulfur and oxygen leads to an uneven distribution of electron density in the SO2 molecule.
The oxygen atoms pull electron density towards themselves, creating partial negative charges, while sulfur carries partial positive charges.
As a result, SO2 exhibits polarity, with a partial negative charge on the oxygen atoms and a partial positive charge on the sulfur atom.
3. Dipole moment
The dipole moment measures the polarity of a molecule. It indicates the separation of charge within the molecule.
To calculate SO2‘s dipole moment, we consider the magnitude and direction of the partial charges on the atoms.
In SO2, oxygen pulls electron density towards itself, creating a partial negative charge, while sulfur carries a partial positive charge. This creates a dipole moment, pointing from the positive sulfur atom towards the negative oxygen atoms.
The calculated dipole moment of SO2 is approximately 1.62 Debye units. This dipole moment is significant because it indicates the degree of asymmetry in the electron distribution within the molecule.
Polar bonds in SO2
In SO2, the bonds between sulfur and oxygen atoms are polar. This polarity arises because oxygen is more electronegative than sulfur, causing it to attract electrons more strongly.
These polar bonds contribute to the overall polarity of the SO2 molecule. The unequal sharing of electrons in the bonds results in partial positive and partial negative charges on the atoms involved.
Specifically, the oxygen atoms carry a partial negative charge, while the sulfur atom carries a partial positive charge.
This unequal distribution of charge creates a dipole moment within the molecule, with a net direction from the positive sulfur atom toward the negative oxygen atoms.
Intermolecular forces in SO2
Van der Waals forces, which encompass dispersion forces and dipole-dipole interactions, play a significant role in SO2.
Dispersion forces occur due to temporary fluctuations in electron distribution within molecules, resulting in temporary dipoles.
In SO2, dispersion forces arise between neighboring molecules due to the movement of electrons, contributing to its overall intermolecular attraction.
Additionally, dipole-dipole interactions occur between polar molecules like SO2. The positive end of one SO2 molecule is attracted to the negative end of another, enhancing intermolecular attraction.
These intermolecular forces influence various properties of SO2, including its boiling and melting points, solubility, and phase transitions.
Molecular polarity
SO2‘s polarity is determined by its structure and bond polarity. The molecule’s bent shape, resulting from the lone pairs of electrons on the sulfur atom, contributes to its overall polarity.
Additionally, the polar bonds between sulfur and oxygen atoms, due to the electronegativity difference, further enhance its polarity.
Various factors influence SO2‘s molecular polarity. The asymmetrical distribution of electron density within the molecule, caused by the presence of lone pairs and polar bonds, plays a significant role.
Additionally, the magnitude of the electronegativity difference between sulfur and oxygen atoms affects the degree of polarity. Furthermore, the molecular geometry and resonance hybridization contribute to the overall polarity of SO2.
Physical properties of SO2
1. Melting and boiling points
Due to its polarity, SO2 has relatively low melting and boiling points compared to non-polar molecules of similar size.
The polar bonds and asymmetrical electron distribution result in weaker intermolecular forces, making it easier for SO2 molecules to break free from each other, hence requiring less energy to change phases.
2. Solubility
SO2 is soluble in polar solvents such as water due to its polar nature. The partial charges on the sulfur and oxygen atoms enable them to interact with water molecules, facilitating dissolution.
This solubility plays a crucial role in atmospheric processes and environmental chemistry, affecting SO2‘s transport and fate in aqueous systems.
3. Density
The polarity of SO2 also affects its density. While it is denser than air, its density is relatively low compared to non-polar gases.
The polarity contributes to its molecular arrangement, impacting the packing density of SO2 molecules in the gas phase.
4. Reactivity
The polarity of SO2 also influences its chemical reactivity. The partial positive charge on the sulfur atom and partial negative charges on the oxygen atoms make SO2 susceptible to nucleophilic attack, particularly by species with lone pairs of electrons.
This reactivity plays a crucial role in various chemical processes, such as its involvement in atmospheric chemistry, industrial synthesis, and environmental degradation.
These physical properties of SO2 are essential for various applications, including air quality monitoring, industrial processes, and atmospheric modeling.
By considering the impact of polarity on melting and boiling points, solubility, and density, scientists can better predict and control the behavior of SO2 in different contexts, ultimately contributing to environmental and public health initiatives.
How does the polarity of SO2 affect its reactions?
Combustion reaction
When SO2 reacts with oxygen (O2), it forms sulfur trioxide (SO3). The polar nature of SO2 allows it to participate in this combustion reaction.
SO2 + O2 → SO3
Acid-base reaction
SO2 dissolves in water (H2O) to produce sulfurous acid (H2SO3). The polarity of SO2 facilitates its interaction with water molecules.
SO2 + H2O → H2SO3
Redox reaction
SO2 undergoes oxidation by reacting with oxygen to form sulfur trioxide (SO3). The polar bonds in SO2 contribute to this redox process.
2SO2 + O2 → 2SO3
Precipitation reaction
SO2 reacts with calcium hydroxide (Ca(OH)2) to yield calcium sulfite (CaSO3) as a solid precipitate. The polarity of SO2 influences its solubility and reactivity.
SO2 + Ca(OH)2(aq) → CaSO3(s) + H2O(l)
Environmental implications
Air pollution
SO2 is a major contributor to air pollution, primarily from industrial activities and fossil fuel combustion.
When released into the atmosphere, SO2 reacts with other pollutants to form harmful compounds like sulfuric acid and sulfate aerosols, contributing to acid rain and smog formation.
Health effects
Inhalation of SO2 can cause respiratory issues such as coughing, wheezing, and shortness of breath. Prolonged exposure to elevated levels of SO2 can exacerbate respiratory conditions like asthma and increase the risk of respiratory infections.
Ecological damage
Acid rain formed when SO2 reacts with water vapor in the atmosphere, can harm ecosystems by leaching nutrients from soil, damaging vegetation, and acidifying water bodies.
This can have detrimental effects on aquatic life, soil fertility, and biodiversity.
Climate change
Sulfate aerosols formed from SO2 emissions can act as cloud condensation nuclei and reflect sunlight, potentially offsetting some of the warming effects of greenhouse gases.
However, this also contributes to regional cooling and alters precipitation patterns, further complicating climate dynamics.
Applications of SO2 polarity
Chemical industry
SO2‘s polarity makes it useful in chemical synthesis processes. It is commonly employed in the production of sulfuric acid (H2SO4), a vital chemical used in various industrial processes, including fertilizer production, metal refining, and chemical manufacturing.
Food industry
SO2 is utilized as a preservative in the food industry due to its antimicrobial properties. It helps inhibit the growth of bacteria and fungi in food products, extending their shelf life and maintaining quality.
Environmental monitoring
The polarity of SO2 makes it a valuable indicator of air quality. Monitoring SO2 levels in the atmosphere helps assess pollution levels, identify sources of emissions, and track compliance with regulatory standards for air quality.
Analytical chemistry
SO2‘s polar nature enables its use as a reagent in analytical chemistry techniques. It is employed in methods such as chromatography and spectrophotometry for the detection and quantification of various compounds in samples.
Research and development
Scientists utilize SO2‘s polarity in research and development activities across various disciplines. It serves as a model molecule for studying molecular polarity, intermolecular interactions, and atmospheric chemistry processes.
Safety precautions and handling of SO2
1. Proper ventilation
Always work in well-ventilated areas to minimize exposure to SO2 gas. Use fume hoods or ventilation systems to remove any released gas from the workspace.
2. Personal protective equipment (PPE)
Wear appropriate PPE, including goggles, gloves, and a lab coat, to protect yourself from contact with SO2. Respiratory protection may be necessary if working with high concentrations of SO2 gas.
3. Handling and storage
Handle SO2 containers carefully to avoid leaks or spills. Store SO2 cylinders securely in a well-ventilated area away from heat sources and incompatible materials.
4. Emergency procedures
Familiarize yourself with emergency procedures in case of accidental exposure or release of SO2. Know how to evacuate the area and seek medical attention if necessary.
FAQs
Why is SO2 considered polar?
SO2 has a bent molecular geometry, with oxygen atoms pulling electron density towards themselves, resulting in partial negative charges, while sulfur carries partial positive charges, creating a dipole moment.
How does SO2‘s polarity affect its properties?
SO2‘s polarity influences its physical and chemical properties, including its solubility in polar solvents like water, its role in acid rain formation, and its reactivity in various chemical reactions.
Why is CO2 nonpolar and SO2 is polar?
The polarity of molecules is determined by their molecular geometry and charge distribution. CO2 is nonpolar due to its symmetrical arrangement of polar bonds, while SO2 is polar because of its asymmetrical structure and uneven charge distribution.
What factors determine SO2‘s polarity?
The polarity of SO2 is determined by its molecular geometry, the bond polarity between sulfur and oxygen atoms, and the electronegativity difference between these atoms.
Conclusion
Understanding SO2‘s molecular structure, bond polarity, and environmental implications is essential for addressing its role in atmospheric chemistry, air pollution, and environmental degradation.
By leveraging the knowledge of SO2 polarity, effective strategies can be developed for pollution control, enhance air quality monitoring efforts, and promote sustainable practices in industries where SO2 is utilized.
Additionally, prioritizing safety precautions and proper handling procedures when working with SO2 is paramount to safeguarding human health and the environment.
Also, learn about the polarity and molecular geometry of CO2.
Thanks for reading.