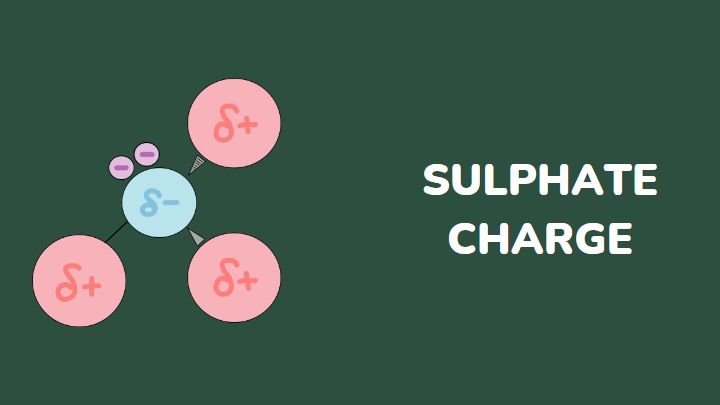
SO4, sulfate, is a radical with a negative charge. It is also known as the salt of sulphuric acid. This salt has various purposes that range from cooking and dietary supplements to industrial use in the production of hair and skincare products.
Sulfate exists alone with a charge and in combination with different metals. Some of the forms in which sulfate exists are magnesium sulfate, copper sulfate, sodium sulfate, hydrogen sulfate, lead sulfate, etc.
In all of these compounds, sulfate has a charge. And sometimes, this charge varies with compounds. Let’s find the natural charge of sulfate and the various charges it can exhibit.
What is the charge of SO4?
The charge of SO4 is -2. This -2 charge on this anion comes from the oxygen bonds that form with sulfur. In dissociation, SO4 is formed by S=O bonds (with two oxygen atoms) and S-O- bonds (with the other two oxygen atoms).
The chemical structure and the charge arrangement on this radical make it work like a ligand.
What is the charge of (SO4)3?
The overall charge of (SO4)3 is -6. The charge of one sulfate molecule is -2 and there are three sulfate molecules which will yield an overall charge of -6.
What is the charge of sulfate in magnesium sulfate?
The sulfate in magnesium sulfate (MgSO4) has a -2 charge.
What is the charge of sulfate in copper sulfate?
SO4 in copper sulfate (Cu SO4) has a -2 charge. This -2 charge neutralizes the +2 charge on copper while the bond forms to create this sulfate salt.
What is the charge of sulfate in sodium sulfate?
The charge of sulfate in sodium sulfate (Na2SO4) is -2. Na has a +1 charge. Therefore, it requires two sodium ions to balance the -2 charge on sulfate.
What is the charge of sulfate in hydrogen sulfate?
The overall net charge on hydrogen sulfate (H2SO4) or sulfuric acid is -1. But, the charge of the sulfate ion is -2.
What is the charge of SO4 in Fe2(SO4)3?
Iron (III) sulfate, Fe2(SO4)3, has two iron and three sulfate ions. One sulfate ion has a -2 charge. Therefore, the three sulfate ions will have an overall charge of -6.
What is the charge of SO4 in Pb(SO4)2?
The charge of one sulfate ion is -2. Lead sulfate, Pb(SO4)2 has two sulfate ions that give an overall charge of -4.
FAQs
What is the formal charge of S in SO42-?
The formal charge of S in SO42- is zero.
What is the charge of S in SO2?
The charge of S in SO2 is +4. Each of the oxygen atoms has a charge of -2 and an overall charge of -4. This is the charge of sulfur when it goes over the equality sign.
Can sulfate be a cation?
No, it cannot. Sulphate cannot be a cation because its overall charge is -2. The sulfate anion is made of one central sulfur atom and four oxygen atoms.
The sulfur atom has a +6 valency while the four oxygen atoms have an overall valency of-8. The sum of this is the overall -2 charge on the sulfate ion.
Conclusion
SO4 is a radical anion with a -2 charge. In most compounds that it forms, sulfate retains its -2 charge. The oxygen atoms in the radical are responsible for the negative charge.
This oxygen and the overall negative charge are reasons why sulfate easily binds to metals to form sulfate salts.
Check out this comparison of sulfate vs sulfite to help you differentiate these two sulfur-based compounds.
Thanks for reading.