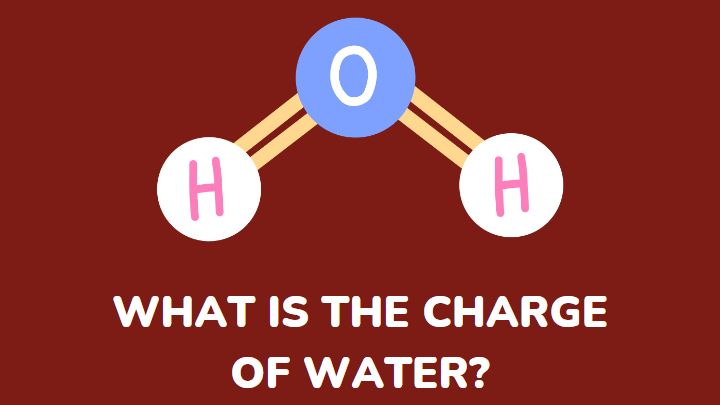
Water is a neutral compound. It is neither acidic nor basic. It does not classify as a negatively or positively charged compound. However, in some representations, a charge may appear on a molecule of H2O.
Generally, water is not a charged compound. But it attracts other water molecules and polar compounds. This phenomenon is possible with charges on compounds.
So, is there a possibility that water carries a charge? Find out in this article.
Properties of water
- Water is a colorless, odorless, and tasteless liquid at room temperature without any interference
- Water is a great solvent
- It has a melting point of 32°F (0°C) and boiling point of 212°F (100°C)
- Water has a high specific heat capacity, latent heat of vaporization, thermal conductivity, and surface tension
- Furthermore, although water tends to appear blue, it is not. This color is a result of its weak absorption bands at wavelengths of 750nm
What is the charge on H2O?
The charge on H2O is 0. Water is a neutral molecule. However, it has partial charges on the hydrogen and oxygen atoms. The two hydrogen atoms have partial positive charges while the oxygen atom has a partial negative charge.
Water is a polar molecule that acts as a magnet. It is drawn to water molecules and polar molecules.
What is the charge distribution in water?
The charge distribution of water shows a positive charge on the hydrogen atoms and a negative charge on the oxygen atoms.
FAQs
What is the charge of H2O as a ligand?
The charge of water as a ligand is 0.
Is water basic or acidic?
Water is neither acidic nor basic; it is neutral. Water is neutral because there is a balance between the hydroxyl and hydronium ions.
Why is water called a polar molecule?
Water is considered a polar molecule because it has an uneven distribution of electrons among the hydrogen and oxygen atoms.
The electrons of the hydrogen atoms are pulled towards the electrons of the oxygen atom. The unequal sharing of atoms and the pull gives the molecule an unsymmetrical shape that takes the form of two poles.
Conclusion
Water is a polar molecule not a charged molecule. Water has partial positive and negative charges, making it attract water and polar molecules.
The charge distribution in water is such that the hydrogen molecules are partially positively charged while the oxygen molecules are partially negatively charged.
Do you know what type of process boiling water undergoes? You should learn about that too.
Thanks for reading.