There are several “twin compounds” in Chemistry and it’s not strange if you are also mixing them up. Among the lot of them are silicone & silicon, ammonia & ammonium, sulfate, and sulfite. These compounds may have some similarities but they are different from each other.
This article discusses a comparison of ammonium vs ammonia. These compounds differ in their chemical formula, structure, melting & boiling points, degree of toxicity, etc. When you know these differences, it becomes easier to differentiate them in reactions and just by seeing their properties.
Read on to see full details.
What is ammonia?
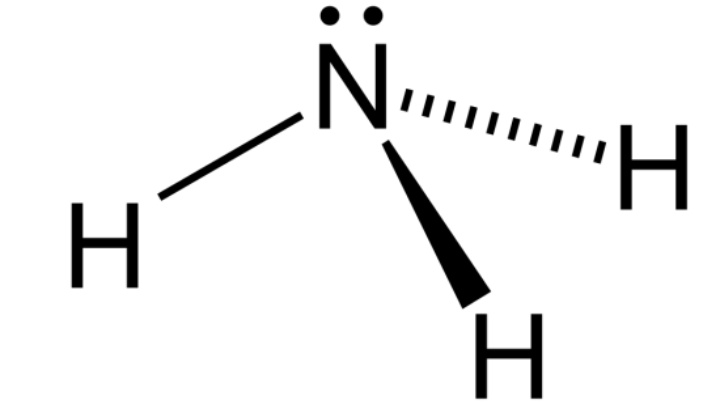
Ammonia (NH3) is a compound of one nitrogen atom and three hydrogen atoms. It is the simplest nitrogen compound and is stable at room temperature.
Ammonia gas has a molar mass of 17.031 g/mol. It is a naturally occurring gas but it can also be prepared in the laboratory.
During the laboratory preparation, the gas is dried by passing it through quick lime and it is collected by the downward displacement of air in a gas jar because it is lighter than air. Also, it is collected over water, like many other gases, because it is highly soluble.
Beyond the laboratory, ammonia is a biologically important molecule. It is a nitrogenous waste from aquatic organisms that contributes to the nutrition of terrestrial organisms. It is a precursor to about 45% of the foods and fertilizers in the world.
What is ammonium?
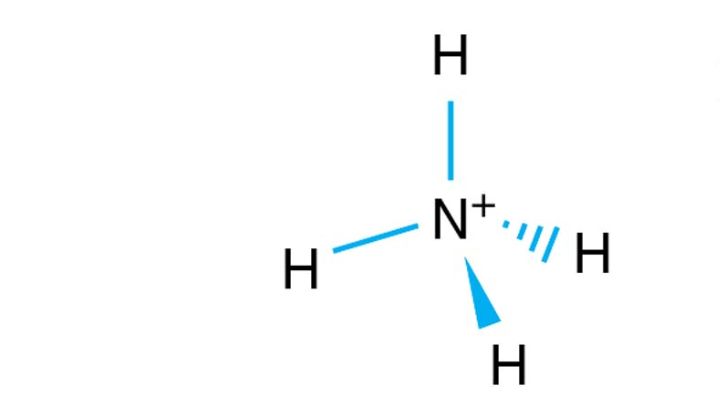
Ammonium (NH4+) is a polyatomic cation formed by the protonation of ammonia. It has a molar mass of 18.039 g/mol.
Ammonium is a product of the reaction between ammonia, a weak base, and Brønsted acids. Brønsted acids are proton donors that transform neutral ammonia to charged ammonium. When ammonia dissolves in water, some of the ammonia gets converted to ammonium.
The degree to which ammonium is formed from ammonia largely depends on the pH of the solution. The reaction produces more ammonium ions when the pH is low and the equilibrium shifts to the right.
Ammonia vs ammonium: Similarities
- Both molecules contain nitrogen and hydrogen atoms
- Ammonia and ammonium have coordination numbers of 4. This means that they can form four coordinate bonds with a central metal ion
- Ammonia and ammonium salts are soluble in water
- In addition, both molecules can participate in hydrogen bonding
Ammonia vs ammonium: Differences
Physical properties
Ammonia is a neutral molecule that exists as a gas at room temperature and atmospheric pressure. But at low temperatures and high pressures, it becomes a liquid. On the other hand, ammonium ion is a cation but is not a gas.
Considering their molecular shapes, ammonium has a tetrahedral structure and ammonia is a trigonal pyramid. The structure of ammonium ions makes it a non-polar molecule. All hydrogen atoms lie symmetrically to the central nitrogen atom and cancel the polarity of the N-H bond.
In contrast, ammonia is a polar molecule because of the uneven distribution of charges on the nitrogen and hydrogen atoms.
Unlike ammonia, ammonium cannot be described by physical properties such as boiling point, melting point, density, solubility, and shape because it is an ion. Moreover, it does not exist by itself. It is found in salts or other compounds, in combination with other elements.
For instance, the solubility, melting point, boiling point, and density of ammonium nitrate will differ from that of ammonium sulfate, and ammonium chloride.
Chemical properties
Considering a comparison of the chemical properties of ammonia vs ammonium, ammonia is a weak base and ammonium is a weak acid. Ammonium has an acidity of 9.25 while ammonia has an acidity of 32.5.
Ammonia is toxic and ammonium is a non-toxic cation. Also, ammonia is generally more compressible than ammonium. However, compressibility will depend on the state of matter of both molecules.
Within the structure of ammonia, there are covalent and hydrogen bonds. Ammonium ions consist of only ionic bonds.
Reactions
Ammonia is stable in the presence of heat and light but it is very reactive. It reacts violently and explosively with halogens and hypochlorites and vigorously with acids and water. It is corrosive to copper, zinc, brass, tin, and galvanized steel surfaces, and forms explosives with silver and mercury.
Furthermore, ammonia is a nonflammable gas but will ignite within vapor concentration limits of 15-28% if the temperature gets as high as 1,204°F (651°C). The complete combustion of ammonia produces nitrogen oxides and water vapor.
Ammonium ion reacts with anions like chloride and sulphate ions to form ammonium salts which have industrial and biological applications. Ammonium ion forms complexes in coordination compounds and reacts with strong bases to form ammonia.
In addition, it is very easy to detect ammonia, even without chemical reactions. Its pungent smell easily gives it away in a room. You can also test for ammonia gas with moistened red litmus paper (it turns blue).
On the other hand, you can detect ammonium ions in a solution by adding dilute NaOH and heating it until pungent ammonia gas is released.
Applications
Here’s another comparison of ammonia Vs ammonium in terms of their applications.
Ammonia is a source of nitrogen for many industries. It is used in the production of fertilizers, paper, leather, rubber, explosives, pesticides, household cleaning products, cosmetics, and compounds like nitric acid, amino acids, urea, and ammonium carbonate.
Ammonia is also used in wastewater treatment, refrigeration systems, and in food & beverage industries,
More extensive applications of ammonia are seen in the petroleum industry for counterbalancing acidity in crude oil and preventing corrosion of equipment, extraction of metals in the mining industry, furnace brazing, carbonitriding, nitriding, and atomic hydrogen welding.
Ammonium ions equally have a wide range of applications. They are also a source of nitrogen for plant species growing on hypoxic soils. Ammonium ions are excreted directly into water by fish and aquatic invertebrates. They are then converted to urea in mammals, sharks, and amphibians via the urea cycle.
In reptiles, terrestrial snails, and birds, metabolic ammonium is converted into solid uric acid which is later excreted.
Ammonium nitrate is a major component of fertilizers, herbicides, and insecticides. It is also used in the manufacture of nitrous oxide, as a nutrient in yeast and antibiotics, as an absorbent for nitrogen oxides, and as an oxidizer for rocket propellants.
Ammonium sulfate is an inorganic salt used in the production of soil fertilizers, flame retardants, leather, alum, and food preservatives. It is also used in water filters during water treatment.
FAQs
Can ammonium and ammonia be converted back to each other?
Yes. When ammonia dissolves in water, some portion of it is converted to ammonium. Heating concentrated ammonium salts with sodium hydroxide or calcium hydroxide converts ammonium to ammonia.
Are ammonium and ammonia naturally occurring compounds?
Ammonium is not naturally occurring; it is a product of ammonia. Ammonia, on the other hand, is a naturally occurring gas that is present in the nitrogen cycle and is also released during the decomposition of plants, animals, and animal wastes.
What is the difference between ammonia and urea?
Ammonia and urea are derivatives of nitrogen but ammonia has a simpler chemical formula and structure than urea. Ammonia is more volatile and mobile than urea.
But, both compounds are components of urine, and urea can be commercially produced from ammonia.
Conclusion
The first and easily identifiable similarities between ammonium and ammonia are the nitrogen and hydrogen atoms. However, while ammonium has four hydrogen atoms, ammonia has three. Moreover, ammonium is the ionic form of ammonia.
Ammonia is a colorless gas with a pungent smell. On the other hand, ammonium ions are not gases and are odorless. You can tell ammonium ions are in a solution through the sodium hydroxide test for the choking smell of ammonia but ammonia is easily detected by its pungent smell.
Despite the differences and basic similarities, both compounds are easily converted to each other in water. I hope you found this ammonia vs ammonium comparison helpful.
You can learn further about the charge of ammonium, its formal charge, and its reactions.
Thanks for reading.
